
nitrogen Students Britannica Kids Homework Help
Select the single best answer. Because CH3NH2 can form hydrogen bonds, and O2, cannnot, Because CH3NH2 is an ionic compound, and the ion-ion attactions are much stronger than the dispersion forces between O2 molecules. Because CH3NH2 is soluble in water, and O2 is not. Because CH3NH2 is a liquid, and O2 is a gas.
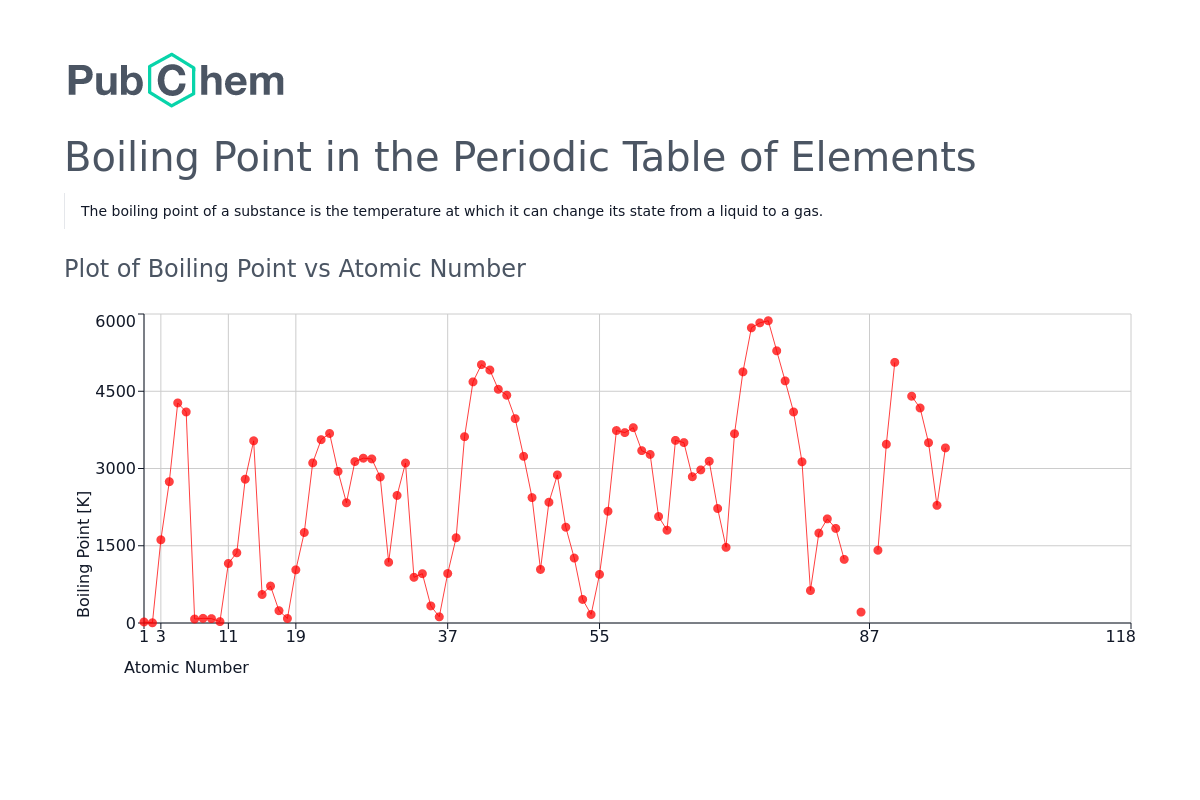
Boiling Point Periodic Table of Elements PubChem
Re: Boiling Point Temps N2, O2, NO. Postby Damian Valle Garcia 3I » Wed Dec 06, 2023 1:05 am. Hello, the order of increasing boiling points is N2 The boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). At sea level, water boils at 100° C (212° F). At higher altitudes the temperature of the boiling point is. Question: The boiling point of O2. The boiling point of O2 is higher than N2 due to. a)dipole-dipole forces. b)dipole-dipole forces. c)ion-ion forces. d)hydrogen bonding. e)ion-dipole forces. There are 2 steps to solve this one. March 30, 2024March 3, 2024by Lambda Geeks. The boiling point of oxygen (O2) is a fascinating topic to explore. Boiling point refers to the temperature at which a substance changes from its liquid state to its gaseous state. In the case of oxygen, this transition occurs at a relatively low temperature. Oxygen has a boiling point of -183 degrees. The reason the boiling point of O2 is higher is not because of increased van der Waals interactions, but simple physics. The mass of a molecule of O2 is greater than that of a molecule of N2, so the molecule of O2 traveling at a speed sufficient to break out of the liquid phase has a greater kinetic energy than an analogous N2 molecule. SO2 has a higher boiling pt because it has a greater molecular mass, SO2 is a pola r molecule which forms dipole-dipole forces which increases the boiling point. So2 has the greater dipole moment. Due to ion Dipole Forces between the atoms or molecules the boiling point of o2 is higher than n2. The electrostatic interaction between an ion and a dipole-containing neutral molecule produces an attractive force known as the ion-dipole force.typically located in solutions. crucial in particular for ionic chemical solutions in polar liquids. Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris) 2.11: Intermolecular Forces and Relative Boiling Points (bp) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The relative strength of the intermolecular forces (IMFs) can be used to predict the. 33. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid to a gas. The stronger the intermolecular forces, the higher the boiling point. Two oxygen molecules are attracted to each other through London dispersion forces. Question: Why does HF have a higher boiling point than O2 ? Explain in terms of intermolecular forces. Select the single best answer. Because HF is a liquid, and O2 is a gas. Because HF is soluble in water, and O2 is not. Because HF can form hydrogen bonds, and O2 cannnot. Because HF is an ionic compound, and the ion-ion attractions are much. Hydrogen Bonding. Page ID. A hydrogen bond is an intermolecular force (IMF) that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons. Intermolecular forces (IMFs) occur between molecules. O2 has a boiling point of -183 degree centigrade while neon has a boiling point of -246 degree centigrade. So, Oxygen has a comparatively higher boiling point than Neon. NO has the highest boiling. Predict which substance of each pair has the higher boiling point and explain. a) nitric acid and nitrogen b) dimethyl ether and ethanol. Why is the boiling point of propane-1,3-diol (HOCH_2CH_2CH_2OH) higher than the boiling point of propane-1,2-diol (HOCH_2CH (OH)CH_3) (215 degrees C vs. 187 degrees C)? Why do both diols have a higher boiling.
The boiling point of water and alcohol explained Noon Academy

The Boiling Point Of Oxygen Is Higher Than Water
Boiling Point คือ อะไร ความรู้และความเข้าใจ

Boiling Point of NH3 and H2O (Explanation of Difference) YouTube

Boiling Point Chemistry Definition DEFINITION GHW
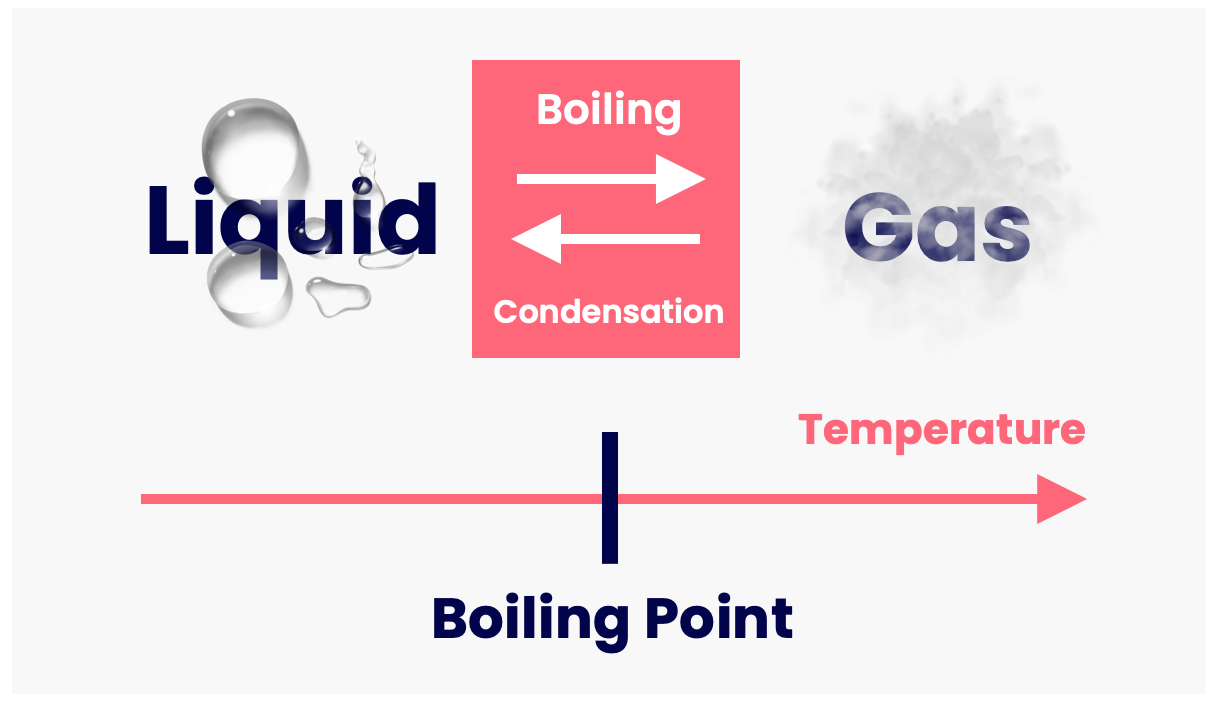
Melting & Boiling • Matter • Physics Fox

Boiling Points And Intermolecular Forces Eshelman Ourighter

Boiling Point Elevation Chemistry Steps
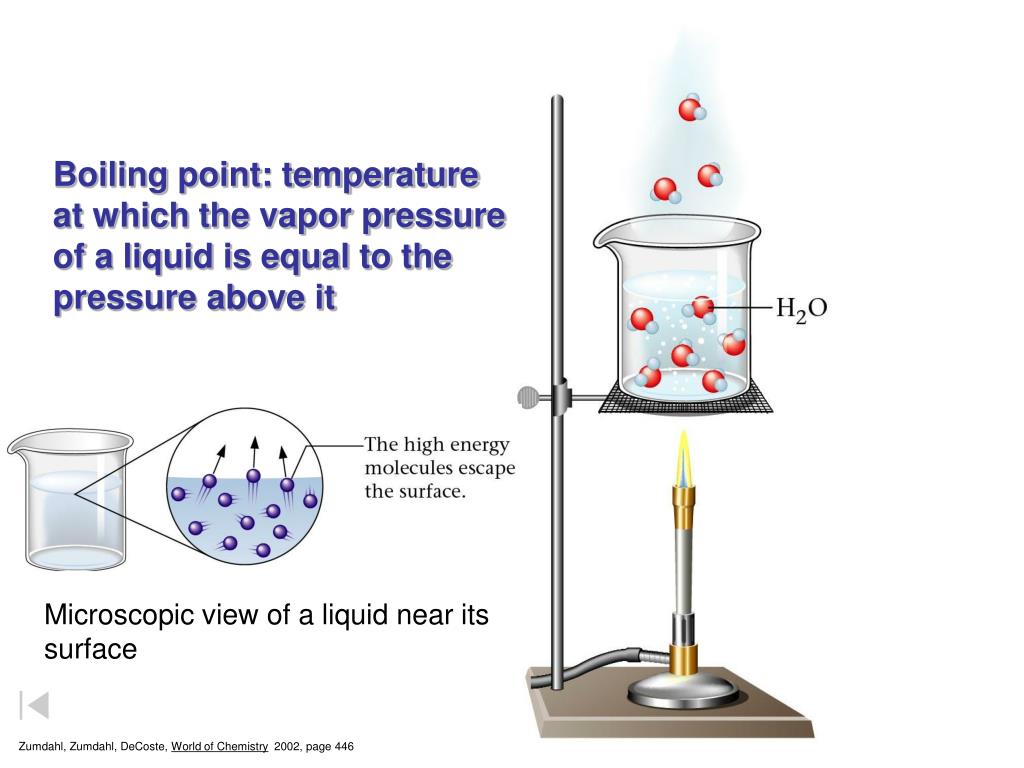
Boiling Point

Boiling Point Elevation Definition and Example

Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling

How to find boiling point of a compound in hysys haasl

Boiling Point Of Water Examples
.PNG)