
Atoms Derekscope
Subatomic particles are particles that are smaller than the atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. An easy way to remember this is to remember that both p roton and p ositive start with the letter " P ." Neutrons have no electrical charge.

Quarks and Leptons Chart Theoretical physics, Elementary particle
subatomic Anything smaller than an atom, which is the smallest bit of matter that has all the properties of whatever chemical element it is (like hydrogen, iron or calcium). About Stephen Ornes. X; Stephen Ornes lives in Nashville, Tenn., and his family has two rabbits, six chickens and a cat.
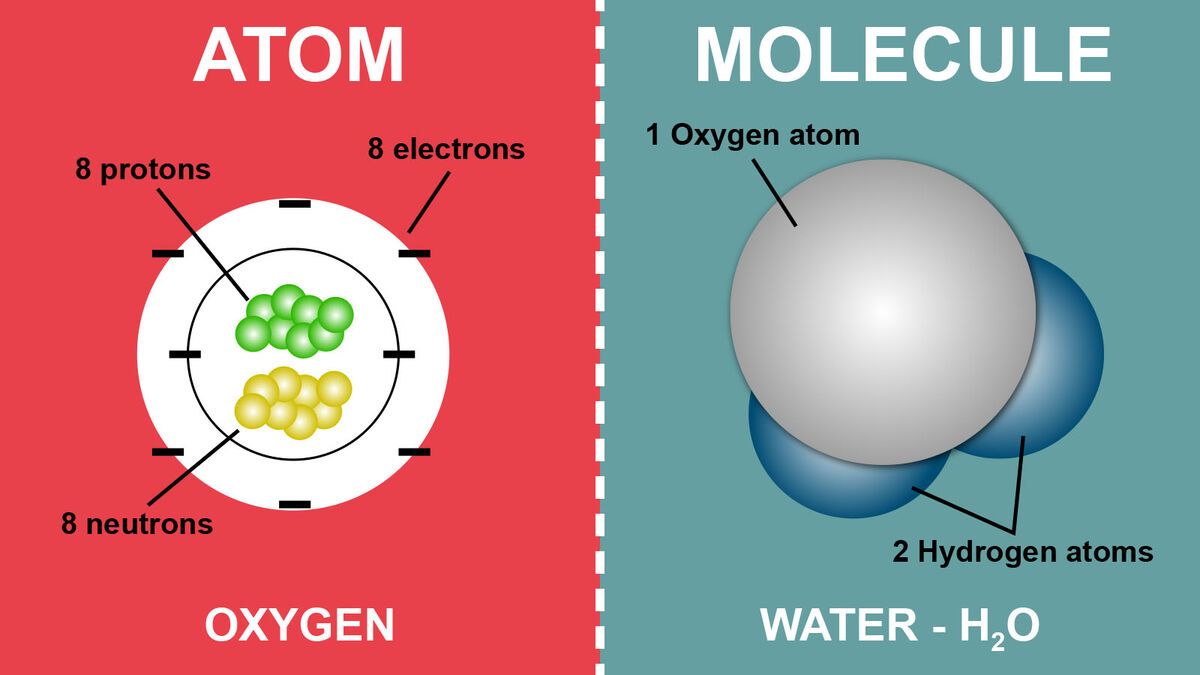
Basic Difference Between an Atom and a Molecule YourDictionary
What are the parts of an atom? Most atoms have three different subatomic particles inside them: protons, neutrons, and electrons.The protons and neutrons are packed together into the center of the atom (which is called the nucleus) and the electrons, which are very much smaller, whizz around the outside.When people draw pictures of atoms, they show the electrons like satellites spinning round.

How Do We Know How Small An Elementary Particle Is? by Ethan Siegel
Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Electrons are found outside the nucleus. Protons are positively charged and have a mass of about 1 u. Neutrons are neutral (have no charge) and also have a mass of about 1 u. Electrons are negatively charged and have a much smaller mass of about 0.0005 u.

PPT Periodic Relationships Among the Elements PowerPoint Presentation
The nucleus contains protons and neutrons; its diameter is about 100,000 times smaller than that of the atom. The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly \(1/12\) of the mass of a carbon-12 atom and is equal to 1.6605 \(\times\) 10 −24 g.
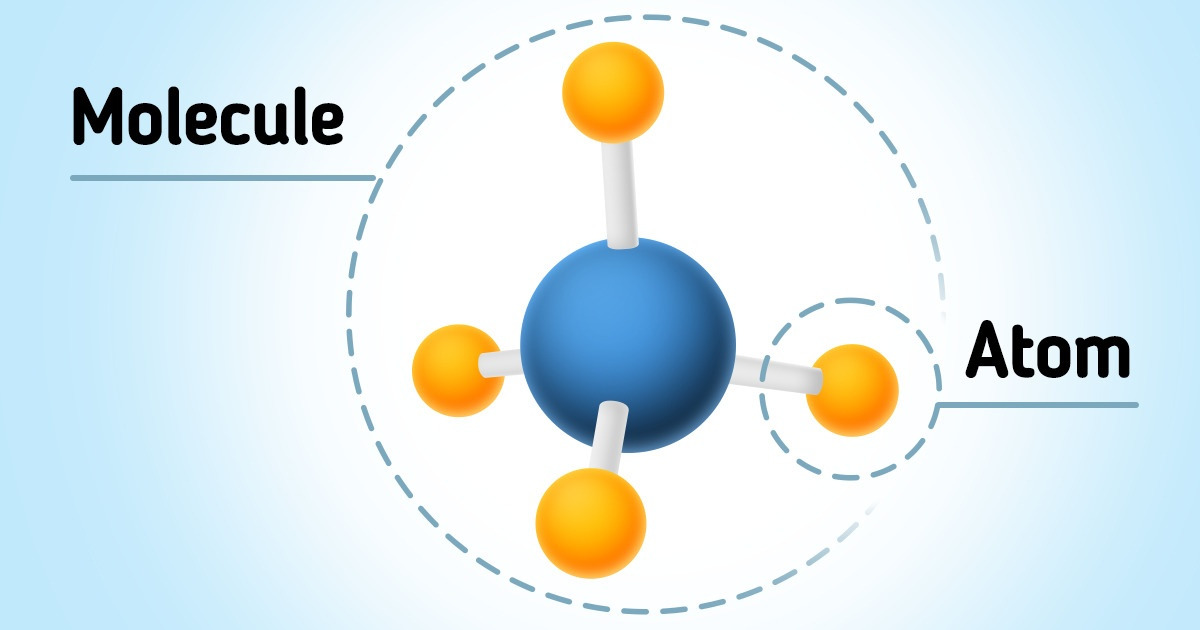
What’s Smaller an Atom or a Molecule? / 5Minute Crafts
subatomic Anything smaller than an atom, which is the smallest bit of matter that has all the properties of whatever chemical element it is (like hydrogen, iron or calcium). superposition (in quantum physics) The ability of some minute subatomic-scale particle to be more than one place at the same time. It has to do with particles in the.
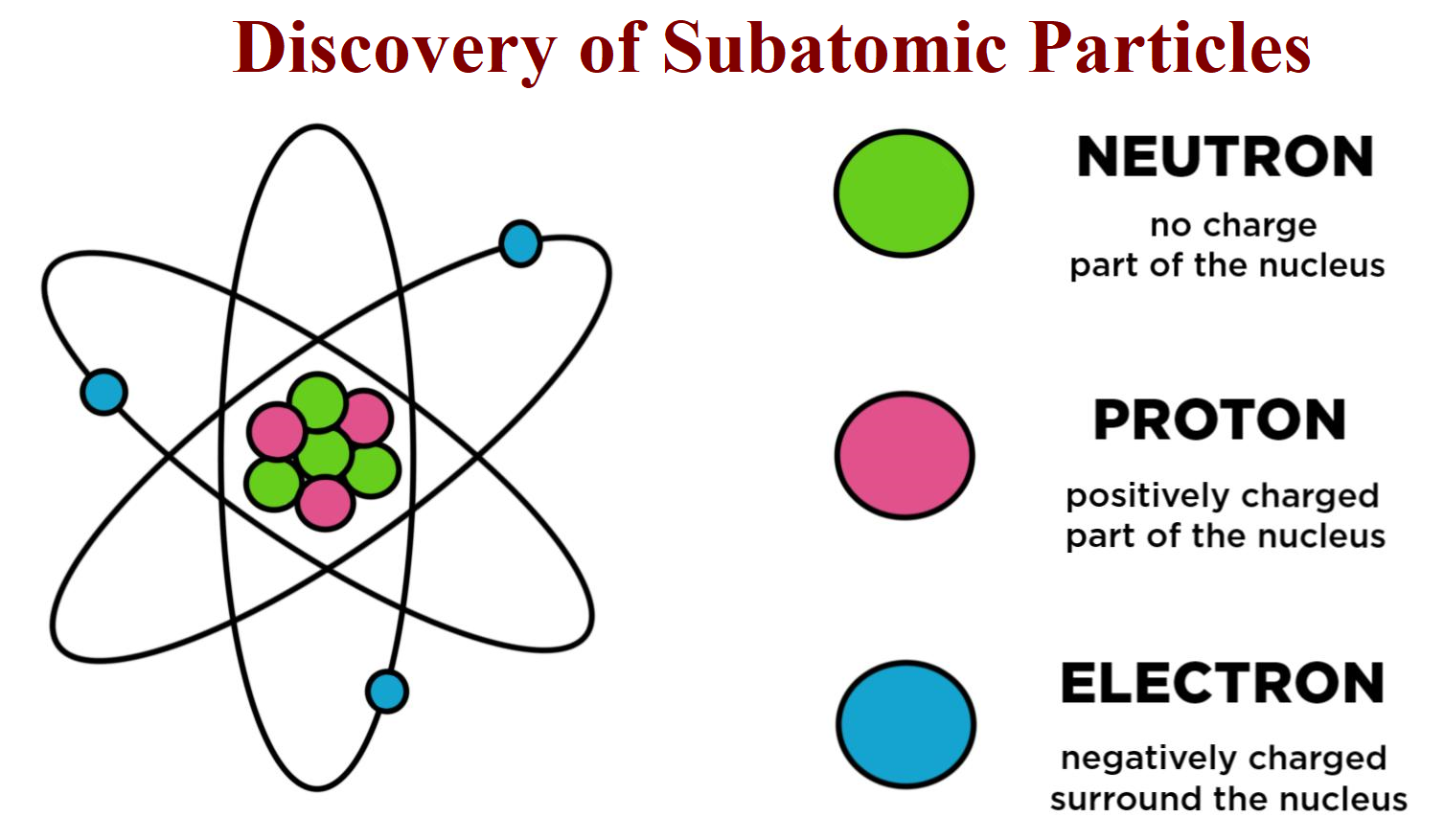
Discovering Subatomic Particles Proton, Neutron and Electrons Gadget
An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, a gold coin is simply a very large number of gold atoms molded into the shape of a coin, with small amounts of other, contaminating elements.. Electrons are much smaller in mass than protons, only about 1/1800 of an atomic mass unit.
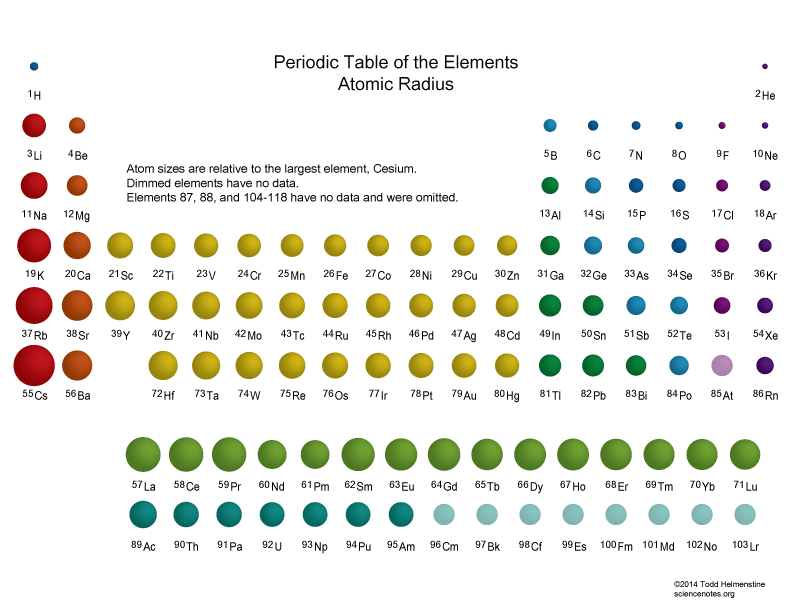
Which are the smallest and largest atoms? Socratic
An atom is composed of a dense central nucleus made up of protons and neutrons, which is surrounded by an electron cloud. Since the electron cloud is much larger than the nucleus, most of an atom is empty space. An atom is about 10 ⁻10 m in size, while the nucleus, at 10 ⁻15 m, is 100,000 times smaller. To put this into perspective, if the.
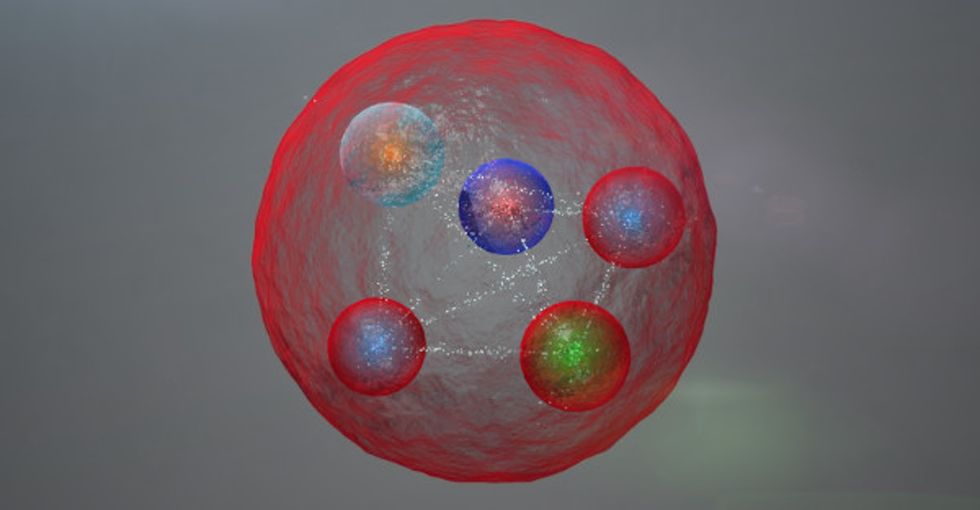
This is the pentaquark. It's smaller than an atom, and until now, no
Electrons. The electron was the first subatomic particle to be identified, discovered by Sir John Joseph Thomson in 1897. Electrons orbit around an atom's nucleus in what is referred to as an electron cloud. The particle's mass is tiny, approximately 1,840 times smaller than protons and neutrons. The subatomic particle has a negative charge.
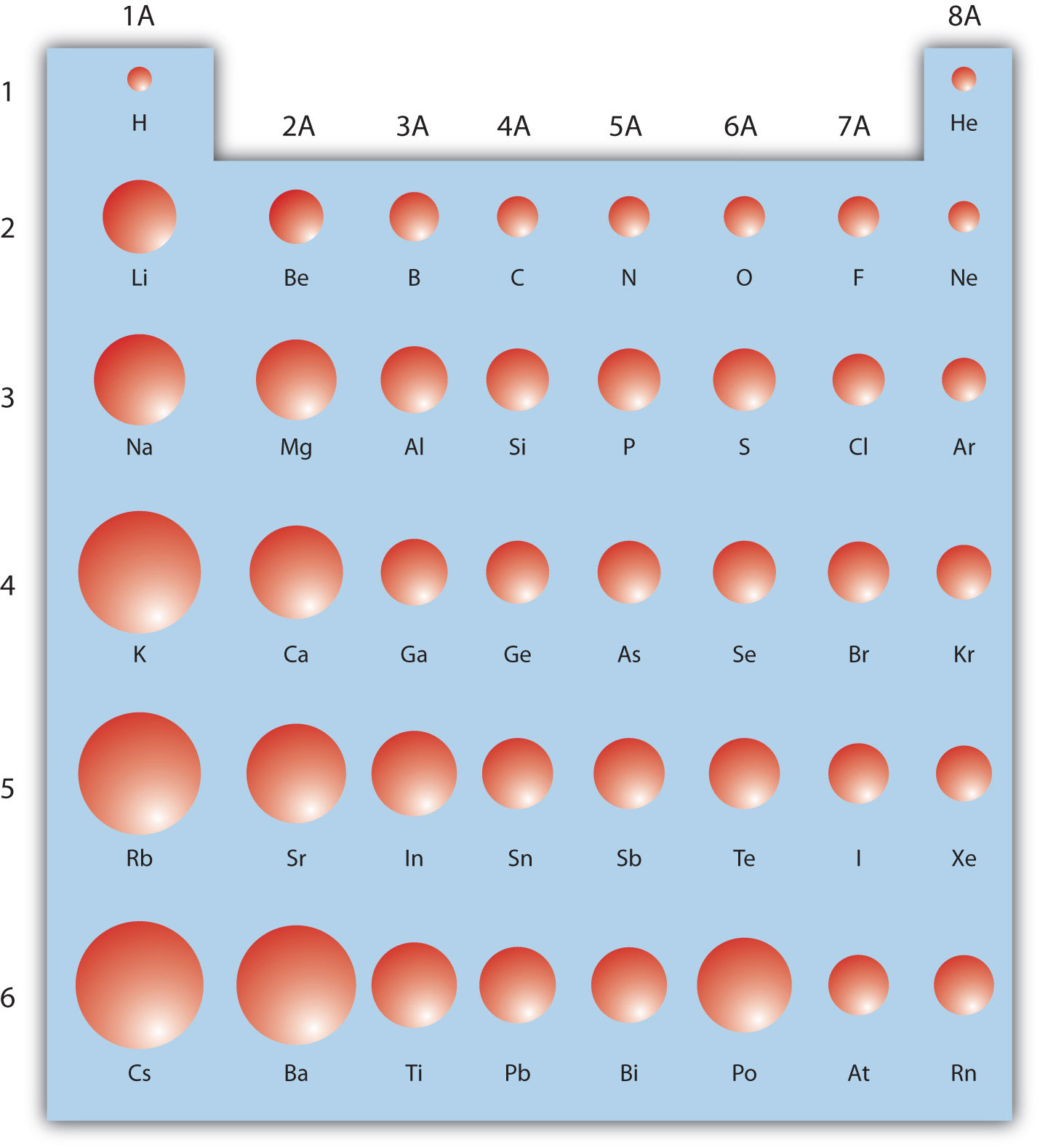
The Periodic Table
The lowest nonzero-mass particle we know of is the neutrino, Lincoln said. He pointed out, however, that we don't have the exact measurement of a neutrino's mass because the instruments used to.
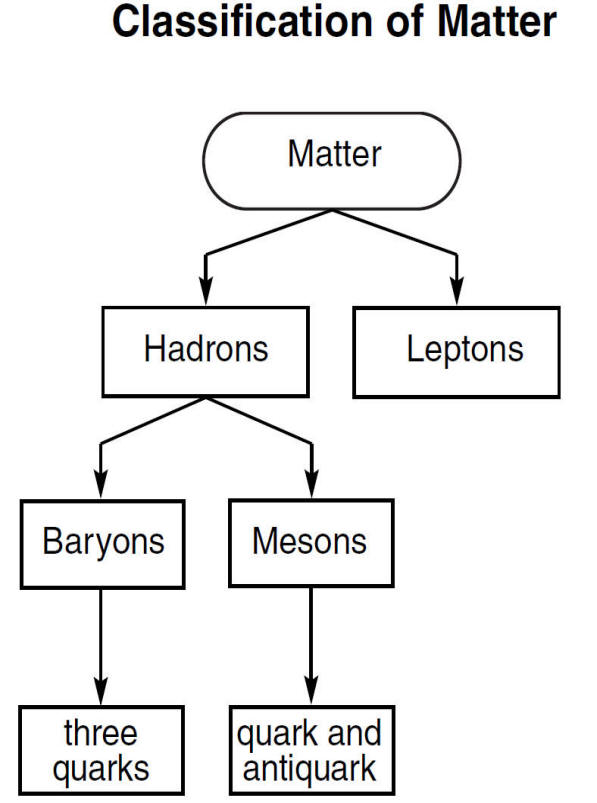
(reference table)
Electrons are tiny—more than 1,800 times smaller than a proton or neutron—and nearly massless. An electron's mass is just \(9.109\,×\,10^{-31}\) kg; that's a relative mass of 0.054% compared to the mass of a neutron. While electrons lack in mass, they make up most of the size of the atom as they can orbit quite far from the nucleus.

Atomic Radius and Ionic Radius
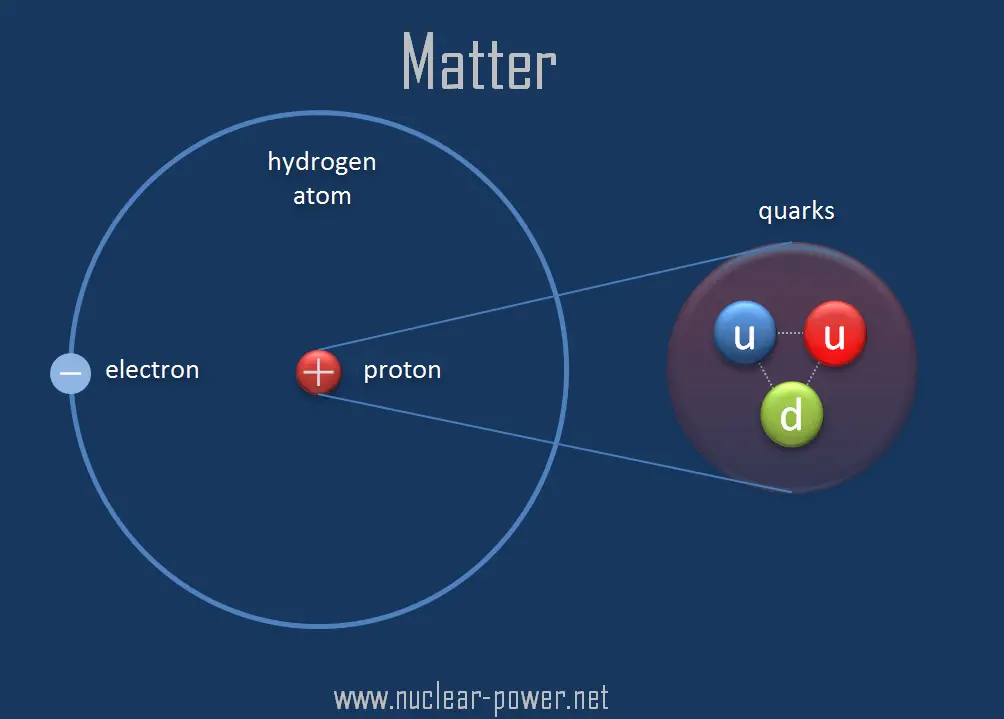
Quark (noun, "KWARK") This is a type of subatomic particle. Subatomic means "smaller than an atom.". Atoms are made up of protons, neutrons and electrons. Protons and neutrons are made of even smaller particles called quarks. Based on the evidence available today, physicists think that quarks are elementary particles.
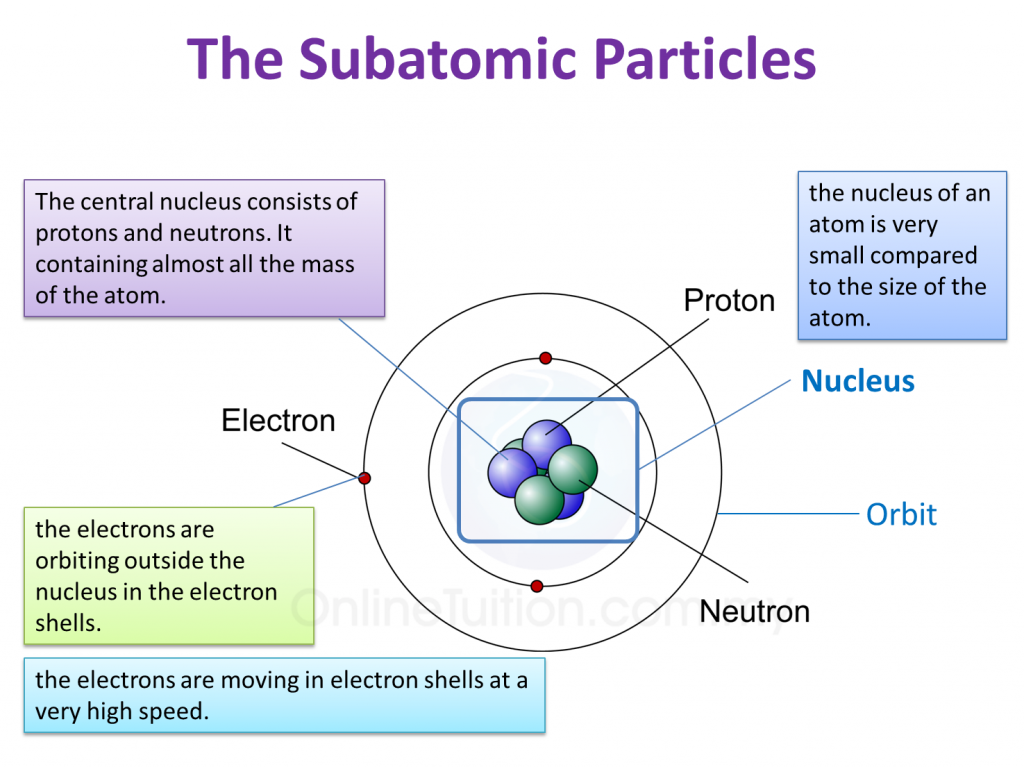
4.2 Structure of Atoms SPM Science
The atom is the smallest and most fundamental unit of matter. Atoms combine to form molecules, which are chemical structures consisting of at least two atoms held together by a chemical bond.. At 0.1-5.0 µm in diameter, most prokaryotic cells are significantly smaller than eukaryotic cells, which have diameters ranging from 10-100 µm.

Zooming In Visualizing the Relative Size of Particles
Subatomic Particles of an Atom. By definition, subatomic particles are smaller than an atom. Atoms consist of three subatomic particles: protons, neutrons, and electrons. Each atom has a nucleus that contains protons and neutrons and has a positive electrical charge. Protons and neutrons are roughly the same mass as one another (neutrons are.

Atomic Radius and Ionic Radius
A subatomic particle is a particle which is part of an atom such as an electron, proton, or a neutron.. The most powerful instrumentation can see objects smaller than a proton, but quarks and.
What are Quarks Definition & Properties
When physicists first collided electrons with protons, they observed that electrons bounced off three small hard nuclei inside the proton. The cores were then called quarks. Quarks are the smallest particles we have come across in our scientific endeavor. The Discovery of quarks meant that protons and neutrons weren't fundamental anymore.